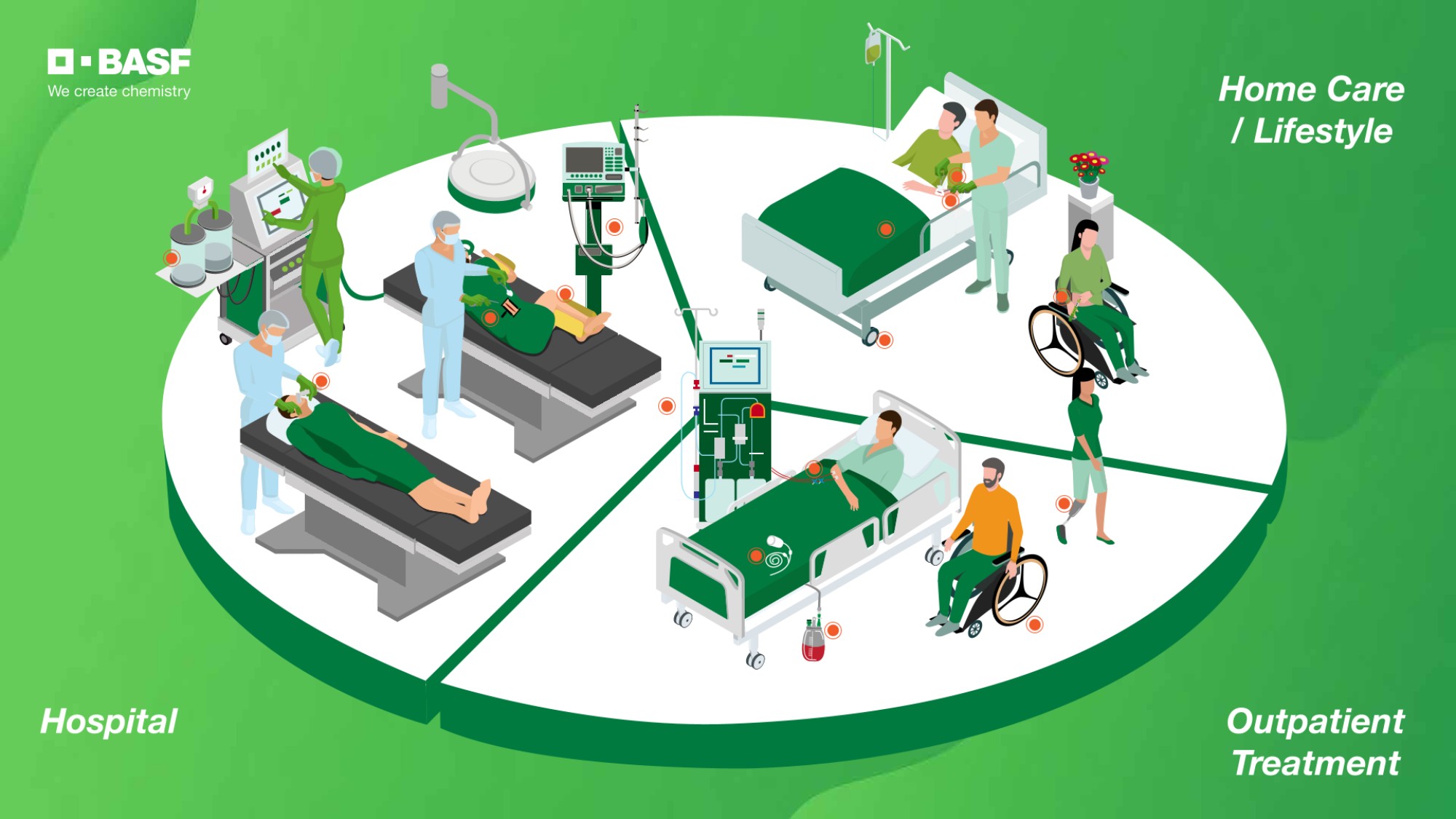
Performance Polymers
Safety for Patients, Reliability for Processors
BASF offers an extensive portfolio of engineering plastics for medical technology needs and understands the increasing customer demands for medical applications by offering high-performance profiles and long-term formulation consistency.

Engineering Plastics with the highest formulation consistency ensure
patient’s safety
Ultraform® PRO (POM) and Ultradur® PRO (PBT) were specifically developed to meet the requirements and needs of the challenging and at the same time risk-averse medical market. The suffix PRO (Profile covered Raw materials Only) points to the fact that only controlled raw materials are being used within the enhanced production process, in combination with testing procedures. Furthermore the service package around the PRO grades assures reliable change control and accordance to regulatory pharmaceutical and medical industry specific expectations. The PRO package fulfills all essential steps for untroubled production of safe medical devices.
Bring us your ideas, we’ll help you turn them into real solutions.
At BASF – Innovation means that we care.
PRO Service package - Safety for patients, reliability for processors
Commitment
- High quality and product purity
- Consistent formulation acc. to Drug Master File (DMF)
- Long-term supply assurance
- Advanced change notification process (36 months)
- Change management procedures
Handling
- Production conditions according to GMP principals*
- Dedicated production line concept and enhanced quality control procedures for raw material and final product
- Back up production line concept (security of supply)
- Sample retention and documentation
- Effective risk management procedures
*Commission regulation (EC) No 2023/2006 of 22 December 2006 on good manufacturing practice for materials and articles intended to come into contact with food

Regulation
- Support of global regulatory approvals for pharmaceutical and medical applications
- Material compliance to FDA and EU requirements
- Certified Biocompatibility (USP Class VI / ISO 10993)
- Drug Master File (DMF) Listing
- Latex free, ROHS, Phthalate free (REACH compliant)

Support
- Increased technical support (color trend, design assistance, analytical testing, calculation (FEM), simulation / filling studies (mold flow), individual part testing, processing guidance, troubleshooting etc.)
- Extensive research and development capabilities
- Testing the compatibility of the plastic to specific chemicals
- Plant audits
- Access to over 50 years of application innovation in different industry areas
Explore our medical applications range with our interactive PDF!
Our medical application range
Our Product Range for Medical Applications
We offer a comprehensive range of products with a broad variety of property profiles. Find the right product for you!
Consult with an expert
Self-service solutions: