Performance Polymers
Opening The Door To Chemistry
Have you recently gone to your local hardware or electronics store looking for an item only to hear the words “unavailable” or “out of stock” from the salesclerk? For businesses, “no inventory” can equate to more formal terms like “force majeure.”
The past several months highlight the domino effect that one disruption in supply can have across an entire value chain. Spurred by recent events, manufacturers are delving deeper and becoming better informed on the upstream components that impact their businesses. One such material used by many door manufacturers and worth a closer look is polyurethanes.
What’s inside door number one?
An entry or garage door may appear simple in construction, but there is a lot that goes into its design. The same is true with polyurethanes; they appear to need only resin and isocyanate for their production. Yet, polyurethanes have a complex chemistry that allows for great versatility. Used to insulate entry doors and garage door panels, polyurethane systems are chosen because of their high insulation value (R-value), strong substrate adhesion, and ability to conform in shape.
Let’s get cracking
Polyurethanes are formed by reacting a polyol (resin) with an isocyanate. At the beginning of the polyurethane process is an oil source or hydrocarbon. Hydrocarbons are composed of hydrogen and carbon atoms bonded in long chains.
The hydrocarbons are fed into a steam cracker. A steam cracker is a large “furnace” where hydrocarbons are “cracked” into smaller, more useful units of either three carbons (propylene) or two carbons (ethylene). These units are now the starting building blocks for new chemical reactions.
Sneakers, seat cushions, and more
The building blocks are then prepared to react in different combinations in order to produce various strength molecules. The longer the molecule, the more flexible it is. Different products require different attributes, including flexibility, durability, and more. Seat cushions is one example where flexible building blocks are used.
Looking for a harder, less flexible material like the one found in door panels? The shorter the molecule, the more rigid it is. The next step involves oxygen. Adding oxygen into the process allows you to create propylene oxide or ethylene oxide; these are highly reactive molecules.
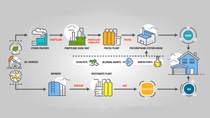
Value Chain of Polyurethane Systems
Under pressure
While under pressure, the propylene oxide or ethylene oxide enters the polyol plant. Before any reaction occurs, there needs to be a starting block or initiator present. An example of an initiator can be sugar. With its strong attraction to sugar, the propylene oxide reacts with the sugar and begins forming chains.
What determines the molecule’s size? The amount of and speed with which the propylene oxide is introduced into the reaction. It is the size of these newly formed molecules, called polyols, that defines the polyurethane’s physical properties.
The same but different
While all these molecules can be grouped as polyols, all polyols are not the same and can vary in sizes and shapes. Polyols made of small, compact molecules have tight linkages that make them ideal for rigid foam such as door insulation. Flexible foam, such as the kind found in seat cushions, will have polyols with larger and longer chains.
Once the polyols are formed, they advance to the polyurethane system house. At the polyurethane system house, other components, such as surfactants, blowing agents, and catalysts, are added to further define what is produced
What's In Polyurethanes?

Lord of the rings
The other side of the polyurethane equation is the formation of isocyanate. At the start of this process, the oil source must be broken into different lengths of hydrocarbons. Some hydrocarbons are straight chains while some are circular in structure (called benzene rings). A benzene ring (six-carbon molecule) is a key building block of organic chemistry and is prevalent throughout nature. The benzene ring goes through additional reactions to form isocyanates like diphenylmethane diisocyanate (MDI). MDI contains two benzene rings, and it is from the different orientations of these rings that the polyurethanes produce their different performance characteristics.
To react or not to react
On both the resin and isocyanate side of the value chain, there are key elements that promote the reactions throughout the processes. One key element is chlorine. Chlorine molecules react very quickly with organic materials.
On the isocyanate side, chlorine’s affinity to react is what helps add the isocyanates to the benzene rings. Without chlorine molecules, the rest of the value chain could not proceed, halting further production of isocyanates, and by default, polyurethanes.
The finale
After all these processes, the final chemical reaction (the actual formation of polyurethanes) occurs when the isocyanate reacts to the resin. For entry doors and garage door panels, the two chemicals are mixed and injected into the door or panel where they react to form the insulating core and adhere the product together.
It’s everywhere
The next time you open the garage door, sleep on your foam mattress, or jog in your sneakers, think about the complexity behind the chemistry — and all the steps it took to reach you. As one of the most versatile products on the market, you don’t need to look too far to find polyurethanes.
BASF’s construction team manufactures polyurethane systems for the residential market including entry and garage doors. For more information, contact market segment manager, Amie Diamond.


